The Primer Designer features a powerful, yet extremely simple, real-time interface to allow the rapid identification of theoretical ideal primers for your PCR reactions. Primer pairs are computed from the set target regions, then screened against a series of parameters to maximise priming efficiency for trouble-free PCR.
The Display
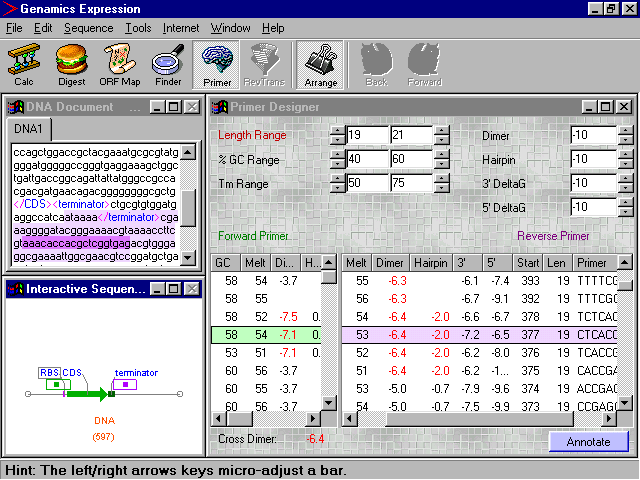
The main Primer Designer form essentially consists of two grids and some parameter controls. The grids show all the possible forward (left side) and reverse (right side) primers that conform to the set parameters, and fall within the specified target regions for each primer. You will immediately notice, that by making the selection conditions for the primers more stringent, the list of possible primers will diminish. This real-time screening process makes it possible to very quickly narrow down the list of potential primers, and select the one(s) that best suit the needs for your experiment.
The regions from which the primers are chosen can be visualised and adjusted on the Interactive Sequence Map. The regions are represented by outlined boxes, colored green for the forward primers and purple for the reverse primers. The regions can be shifted by dragging them with the mouse, and their size adjusted by clicking on their edges and stretching them to the desired size. For fine adjustment of the region's position or size, simply select the whole box or the appropriate edge, then use the left and right arrows to shift your selection to the exact desired position. The primer selection regions are also shown in the Sequence Editor as light green and purple shaded regions.
The positions of the currently selected forward and reverse primers in the tables on the Primer Designer form, are shown on the Interactive Sequence Map as solid green and purple bars, and on the Sequence Editor as darkly shaded green and purple regions.
Adjusting the Primer Selection Parameters
Various parameters can be adjusted on the Primer Designer form, allowing you to specify how the primers will be screened. Although the default settings of the parameters are adequate for a typical PCR reaction, you will probably need to adjust them to some extent depending on the nature of your experiment. The parameters and their functions are outlined in the table below:
| Parameter | Function |
| Length Range | Specifies the minimum and maximum lengths of the primers. |
| %GC Range | Specifies the minimum and maximum percentage of GC content in the primer. Efficient primers generally have %GCs of around 50. |
| Tm Range | Specifies the minimum and maximum melting temperature (in degrees Celcius) of the primer, as calculated by the Nearest Neighbor method (see below). |
| 3' End Stability | Determines the DG of the last 5 bases at 3' end. An unstable 3' end (less negative DG) will result in less false priming. |
| 5' End Stability (GC Clamp) | Determines the DG of the last 5 bases at 5' end. A stable 5' end (more negative DG) will result in more efficient and specific bonding to the template. |
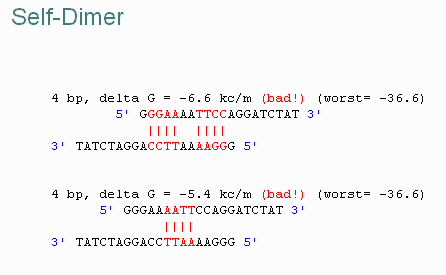
| DG Dimer | Specifies the minimum tolerable DG for primer dimer formation. |
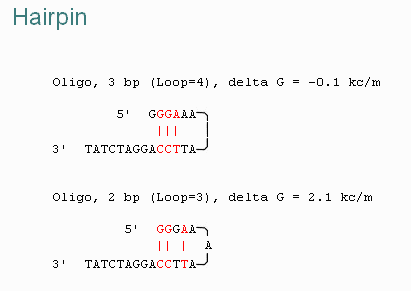
| DG Hairpin | Specifies the minimum tolerable DG for primer hairpin formation. |
In the functions above, all the DG values are calculated according to nearest-neighbor method (Breslaur et al., 1986).
There are three commonly used methods for calculating the Tm. Expression uses the most accurate method, nearest-neighbor. The formulas and references for the different methods are summarised in the table below:
| Method | Formula | Reference |
| Arbitrary | 2(A + G) + 4(G + C) | Wallace et al. (1979) |
| Nearest Neighbor | (-1000 x deltaH) / (-10.8 - deltaS + R x ln(2.5^c/4)) - 273.15 - 16.6(log10 M)
where c is the molar concentration of primer (set at 250 pM), M is the molar concentration of Na+ (set at 50 mM), and R is the gas constant (1.987) | Breslaur et al. (1986); Rychlik et al. (1990) |
| Long probe | 81.5 + 16.6(log10 M) + 0.41(% GC) - 0.61(% form) - 500 / Length in bp
where M is the molarity of Na+ (set at 0.75 M) and % form is the percentage of formamide (set to 50%) | Meinkoth and Wahl (1984) |
Annotating your Primers
Once you have finished refining the stringency of your primer screening, and spotted the primers you would like to use, you can annotate their positions, and that of the PCR product, into your sequence for future reference. To do this, simply highlight the forward and reverse primers of choice by clicking on them on the tables on the Primer Designer form, and push the Annotate button. It really is that easy.
References
Breslaur KJ, Frank R, Blocker H, and Marky LA (1986). Predicting DNA duplex stability from the base sequence. Proc Natl Acad Sci, 83:3746-3750.
Meinkoth J, and Wahl G (1984). Hybridization of nucleic acids immobilized on solid supports. Anal Biochem, 138(2):267-284
Rychlik W, Spencer WJ, and Rhoads RE (1990). Optimization of the annealing temperature for DNA amplification in vitro. Nucleic Acids Res, 18(21):6409-6412.
Wallace RB, Shaffer J, Murphy RF, Bonner J, Hirose T, and Itakura K (1979). Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res, 6(11):6353-6357.
Related Articles
Using the Sequence Map
Annotating Your Sequences
Return to Expression Overview